
Nobel Laureate Decodes Answers about Life’s Beginning, Medicine’s Future

As Nobel laureate and Ohio University alumnus Venki Ramakrishnan painted the big picture of his journey from graduate school in Athens to a molecular lab in Britain, he also showed the tiniest picture of life.
It’s a discovery that won a Nobel prize, helps solve the riddle of how life began, and now can help pharmaceutical companies build better antibiotics.
And that tiny picture of the ribosome at work is both language and machine, combined with a beautiful waltz of dancers looking for the just right pairing.
If you’re a left brain analyzer, you might appreciate a piece of genetic code—made of combinations of four letters in groups of three—that can combine to manufacture the 20 or so proteins needed to build and run the human body.
If you’re a right brain visualizer, you might see a dancer looking for the partner who brings along exactly the right amino acid needed to build a protein chain—rejecting suitors until the right match shows up, and then stepping aside to let the next pair of dancers form.
But the picture Ramakrishnan had in mind was the biggest question in biology—the ribosome, a molecule made up RNA and protein foundational to life. He knew it needed to be seen in order to be understood.
Ramakrishnan described the ribosome and his journey in easy-to-understand conversation with an audience of several hundred who braved a thunderstorm to pack Walter Rotunda and hear the man who earned a Ph.D. in Physics from OHIO describe having to take undergraduate courses to get up to speed as a Biology researcher, then winning the 2009 Nobel Prize in Chemistry.
Copies of his story, Gene Machine: The Race to Decipher the Secrets of the Ribosome, sold out quickly.
A Gene Cookbook for the Body
Ramakrishnan noted that people have about 25,000 genes, but then a worm or a plant might have that many, too.
“Genes are essential information about how to make proteins,” he said. Just as amino acids are the building blocks of proteins, proteins are the building blocks and fuel of the human body.
“Each gene has information to make a particular kind of protein. You can think of a gene as a page in a cook book…. And proteins go off and carry out all sorts of functions. They carry out chemical reactions. They give structure. They form your hair, your skin, etc.”
Proteins in humans are long chains made up of a various combinations of about 20 different amino acids. “So you can have different chains which have different permutations of these 20 amino acids. So you can think of a protein as a kind of sentence where the letters represent the amino acids.”
Like origami on auto-pilot, these protein chains automatically fold up into different shapes—such as collagen in skin tissue, hemoglobin to carry oxygen in the blood, or rhodopsin in the retina to sense light.
“The reason I gave you these three proteins is that they are totally different shapes and totally different functions,” he said, “and there are thousands of these, each having its own shape and its own function. And every one of them is made somehow by reading instructions in our genes.”
A Base Language with Only Four Letters
Genes are made of DNA, and DNA is two intertwined strands made up of just four bases in varying order and varying number.
But form didn’t illuminate function. “What was not clear from the DNA structure was how the order of bases in DNA could somehow specify how to make a protein? So how does that work?” Ramakrishnan said.
“A molecule of DNA in us contains thousands of genes. We have 23 chromosomes, so our 25,000 genes are distributed among our 23 chromosomes. You can think of DNA as a library with lots of books, or a cookbook with lots of recipes.”
He made an analogy to archived books, where the library doesn’t let you check out the original book, but they will give you a copy.
“And the cell does something very similar. When it needs a particular protein, it makes a copy of the gene for that protein. And that copy is like a copy of one of the strands of DNA, and it’s called messenger RNA.”
So where DNA is a double helix, or two strands, messenger RNA is a single strand carrying the same information. There are still just four bases—i.e. four letters—in various orders and amount that carry the recipe for a specific protein.
“Now how is this read?” he continued. How does nature read the code—a language made up of a sequence of bases—and convert it to a sequence of amino acids to form a protein?
In the RNA, researchers label the four bases G, C, A and U. But since it’s hard to spell many words—or the 20 proteins needed—with just four letters, “nature uses a three-letter code. It will read three of these letters as a unit. That unit is called a ‘codon’ because it’s a unit of the genetic code.”
Translating the Code
But something was still missing. Picture an engine running and the wheels turning—what transfers the energy from the engine to the wheel?
Amino acids can’t recognize DNA directly, so scientists began looking for an adapter molecule—one that would pair with three bases on one end (like a three-pronged extension cord plug), and at the other end carry an amino acid building block (like a bindle on a hobo’s stick).
Once discovered, these were called transfer RNA molecules.
“They are called transfer because they are transferring amino acids to the growing protein chain,” Ramakrishnan said.
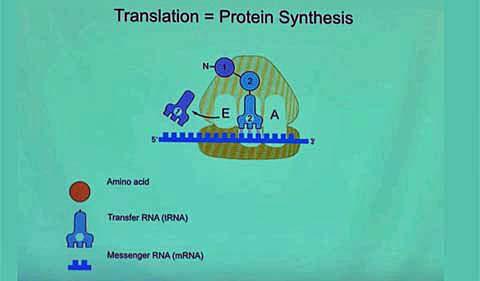
The Protein Factory—the Ribosome
All this protein-building activity is happening in a tiny manufacturing center called the ribosome. Ribosomes are specialized molecules made of about one-third proteins and two-thirds RNA.
- Note: If you don’t have time to watch Ramakrishnan’s May 2 entire talk in Athens, skip ahead to 1:08:40 in the video and watch his movie of how a ribosome works—now that you know about messenger RNA, transfer RNA, amino acids and proteins.
Here’s how Ramakrishnan describes the ribosome’s assembly line, which you can also see in his video:
There are lots of TRNA in the cell, and they can come in at random … and if they don’t match the code, they’re rejected. But if they match the code, the ribosome joins the first two amino acids. And then the whole thing has to move. And then once it moves, the first TRNA is kicked out, the new TRNA becomes the old TRNA, but notice now it has two amino acids attached. And then a third TRNA comes in, and if it’s the right one, it’s accepted and then (its protein) is joined…. The ribosome is only choosing the TRNAs that correspond to the code on the message, and those TRNAs have the right amino acids attached to them, so when it’s stitching together the amino acids, they are in the order specified by the code on the gene.”
“That’s the beauty,” he said, “and that’s why it’s called translation, because it’s translating from the language of genes to the language of proteins.
“So all of this was known when I entered the field.” But to understand a machine like this, you have to see the details, get a closer look, he noted. “You’ve gotta be able to see things to get to that next level of understanding.”

Finding the Right Tools
So Ramakrishnan began his work on the ribosome using X-ray crystallography.
“What you do is you form crystals. And crystals are regular, three-dimensional stacks of molecules…. You then take the crystal and you hit it with a beam of X-rays, and you collect the scattered rays.” And then the computer becomes the lens, refocusing the data into an image.
“If you were able to collect these scattered rays, then if you were somehow able to mathematically recombine those scattered rays in a computer, you would be able to regenerate an image of the object.”
But in theory took a long time in practice.
And he would have to learn X-ray crystallography.
Ramakrishnan was at Brookhaven National Lab using neutron scattering—not X-ray crystallography—to look at biological structures, “but I realized I wasn’t going to be able to solve anything interesting about the ribosome” with neutron scattering. So he took a sabbatical year, on a Guggenheim fellowship, to the MRC Laboratory of Molecular Biology in Cambridge, England—to learn how to use X-ray crystallography to get the pictures he wanted.
Tiny Images But No Big Picture
And he got lots of little pictures.
He was looking at tiny pieces of the ribosome—like unassembled parts of a car with no assembly manual.
That was the state of play in the mid-1990s, he said.
“I had some ideas from my sabbatical about how it might be possible to tackle something as large as the whole ribosome, in terms of getting enough signal in the diffraction experiment.” For starters, he needed good quality crystals.
But now he was a professor with a small lab and an NIH grant at the University of Utah. And he was not alone in pursuing answers to the structure of the ribosome.
He and his team of two graduate students were competing with research teams around the world.
“I found I was going to be in a head-to-head contest with extremely well-funded groups who had been doing it for a long time, and I had my two first-year graduate students in Utah…. I was thinking of giving up the whole thing altogether,” he said.
“But then I thought, ‘Look, crystals have been around for a long time, nobody’s yet figured out how to solve it, and I have ideas.’ And I also thought, ‘This is the most important problem in this field, and one of the most important problems in biology—how a gene is read to make proteins.'”
So back to the lab at Cambridge we went, and 1999 found him with expert colleagues in crystallography and stable funding.
The ribosome has two parts—called the large subunit and the small subunit.
Since others had the large subunit well in hand, Ramakrishnan’s team tackled the small subunit.
World Dominated by RNA Comes into Focus
“Now, when you do the experiment, it doesn’t tell you the structure of the molecule. It gives you a three-dimensional image…. So how do you get an atomic structure from this sort of image? It’s a bit like fitting together a large, three-dimensional jigsaw puzzle,” Ramakrishnan said.
Within about a month of each other in the year 2000, a group at Yale solved the large subunit picture, and the Ramakrishnan group solved the other half of the puzzle, the small subunit.
The picture of both the structure and the function of the ribosome quickly started coming into focus.
“One of the things that came out of it is the place (in the large subunit) where the amino acids are joined together to start making the protein—is in this pocket made up entirely of RNA…. And similarly the part (in the small subunit) which binds the genetic message and where the message is read—is almost entirely RNA.
“Why is this interesting? It’s because there was a long-standing chicken or egg puzzle. The ribosome is what makes proteins, but if the ribosome itself is made of both RNA and proteins, how did the ribosome even come into being?” he asked.
“What we now believe is that life began as a world dominated by RNA—because RNA can carry out chemical reactions but it can also carry information, like DNA. And then it was used to make proteins, and then eventually proteins took over a lot of the chemical reaction and other roles that originally might have been played only by RNA. And DNA probably may have come later.
“And so RNA probably is the oldest information molecule in the world. The structure shows that.
“And then six years later, we solve the entire ribosome”—with messenger RNA, translational RNA and over half a million atoms.
“And if you solve the structure of the ribosome, you can now solve the structure of antibiotics bound to the ribosome,” Ramakrishnan said. “So we were able to solve lots of different antibiotics bound to the ribosome, and drug companies are now trying to use these structure to try and design better antibiotics.
While he was in Athens, Ramakrishnan received an honorary doctorate from his alma mater and gave a technical talk on “Termination of Translation in Bacteria and Eukaryotes.” (If you remember a codon from earlier in this story, his termination talk involved “stop codons,” i.e. reaching the end of the message.)
